尽管对慢性肾脏病矿物质与骨异常(CKD-MBD)的机制与管理策略都已经有了更深入的认识,但是临床上仍然将血磷管理作为慢性肾脏病(CKD)各期患者预后改善的关键一环。然而,由于现有管理策略存在局限性,血磷管理仍然面临着严峻的挑战,高磷血症仍然发生在极大多数透析患者身上。
那么我们为什么要管理高磷血症?如何有效的管理高磷血症?2015年美国学者Jorge B. Cannata-Andía与Kevin J. Martin 在Nephrol Dial Transplant杂志上发表一篇综述,全面回顾了目前对高磷血症危害与管理的认识,本文将结合该综述对高磷血症领域的目前发展做出简要的总结[1]。
我们为什么要管理高磷血症?
心血管疾病是CKD患者死亡的一大诱因,超过50%的终末期肾病(ESRD)患者死于心血管疾病[2]。大量临床研究与实验研究揭示,高磷血症与CKD-MBD的多个不良结局相关,其中包括对心血管系统的危害,如:外周动脉硬化、内皮功能损伤、血管钙化、心血管疾病、以及更高的死亡风险[3-10]。
Block等对超过40000例血液透析患者进行分析发现,血磷水平>5.0mg/dL的患者死亡风险上升(与血磷≤5.0mg/dL的患者相比,血磷水平为5.0-6.0, 6.0-7.0, 7.0-8.0, 8.0-9.0及>9.0 mg/dL的患者相对风险分别为1.07, 1.25, 1.43, 1.67及2.02)[11]。
日本一项纳入128125例血液透析患者的研究显示,仅管理血磷的患者的死亡风险比为1.17,而仅管理钙或PTH的患者的死亡风险比分别为1.41、1.47,提示血磷管理的获益比血钙或PTH管理的获益更多[12],因此多个指南推荐CKD-MBD管理时应优先管理血磷[13-15]。
高磷血症如何导致心血管疾病?
研究显示,高磷可能通过多个途径导致心血管疾病(如图1所示)。血磷水平上升与加速血管钙化进展相关。高磷血症还可直接通过提高活性氧自由基水平,引起氧化性损伤及内皮细胞功能紊乱,从而导致血管功能异常。另外,高磷血症还可间接引起PTH与FGF23水平升高,而PTH与FGF23水平升高与心血管疾病的发生直接相关。血磷升高还与1,25-二羟维生素D合成抑制相关,后者与血管钙化和心肌疾病相关 [16]。
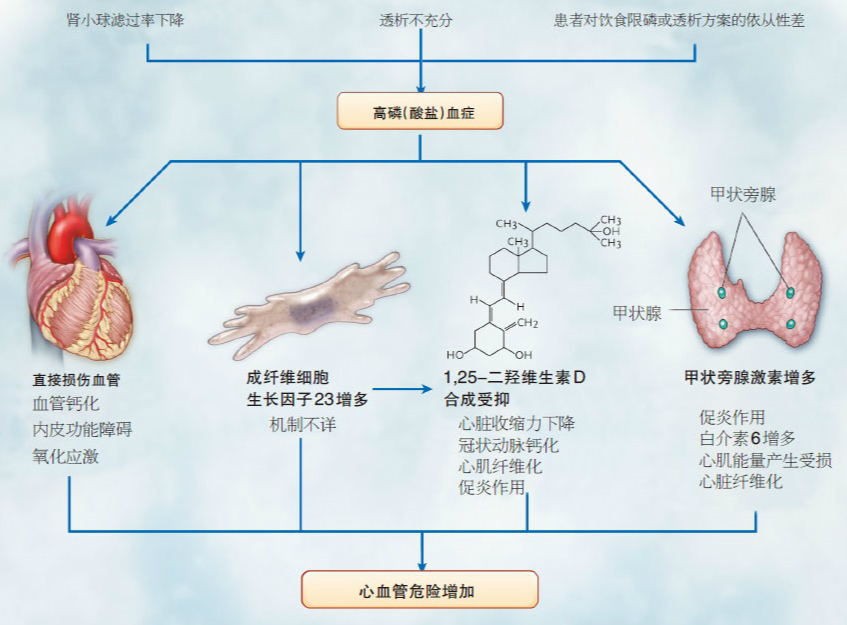
图1:高磷血症导致心血管疾病的可能作用机制
如何有效地管理高磷血症?
基于目前的认知,CKD早期的磷潴留和CKD晚期的高磷血症是CKD-MBD进展的核心环节,因此在CKD-MBD的防治中,对“磷”的干预无疑至关重要。目前针对高磷血症的管理主要有三种途径:限制饮食磷摄入、使用磷结合剂、ESRD患者中保证充分的透析。
然而,由于临床上严格限制磷摄入的难操作性并且可能会使蛋白摄入受限,同时传统一周三次的透析频率仅能清除少量的磷,磷结合剂成为了主要的降磷手段。
表1:常用的磷结合剂
由于铝的毒性,含铝磷结合剂很少用于临床实践中的长期治疗。含钙磷结合剂因其价廉而应用广泛,但越来越多的证据显示使用钙剂会导致正钙平衡。正钙平衡与血管钙化进展加速相关[17]。目前广泛使用的非含钙磷结合剂司维拉姆(盐酸司维拉姆、碳酸司维拉姆)被多项研究证实不仅可有效降低血磷,还可延缓血管钙化进展,提高患者生存率[18-22]。并且研究也发现,司维拉姆对多个心血管疾病的危险因素,如脂质紊乱、氧化应激等具有调节作用[22-25]。
最后,考虑到高磷血症对临床结局的不良影响,在关注磷结合剂降磷效果的同时,还应关注磷结合剂对患者临床预后的改善。也期待大样本、安慰剂对照且设计良好的研究能明确给出磷结合剂降低血磷能带来临床预后改善的证据。
1. Cannata-Andia, J.B. and K.J. Martin, The challenge of controlling phosphorus in chronic kidney disease. Nephrol Dial Transplant, 2015.
2. Giachelli, C.M., Vascular calcification mechanisms. J Am Soc Nephrol, 2004. 15(12): p. 2959-64.
3. Goodman, W.G., Vascular calcification in chronic renal failure. Lancet, 2001. 358(9288): p. 1115-6.
4. Roman-Garcia, P., et al., High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone, 2010. 46(1): p. 121-8.
5. Hruska, K., et al., Cardiovascular risk factors in chronic kidney disease: does phosphate qualify? Kidney Int Suppl, 2011(121): p. S9-13.
6. Huttunen, M.M., et al., High dietary phosphate intake reduces bone strength in the growing rat skeleton. J Bone Miner Res, 2007. 22(1): p. 83-92.
7. Ix, J.H., et al., Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol, 2009. 4(3): p. 609-15.
8. Di Marco, G.S., et al., High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int, 2013. 83(2): p. 213-22.
9. Adeney, K.L., et al., Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol, 2009. 20(2): p. 381-7.
10. Foley, R.N., Phosphorus comes of age as a cardiovascular risk factor. Arch Intern Med, 2007. 167(9): p. 873-4.
11. Block, G.A., et al., Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol, 2004. 15(8): p. 2208-18.
12. Taniguchi, M., et al., Serum phosphate and calcium should be primarily and consistently controlled in prevalent hemodialysis patients. Ther Apher Dial, 2013. 17(2): p. 221-8.
13. Fukagawa, M., et al., Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial, 2013. 17(3): p. 247-88.
14. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl, 2009(113): p. S1-130.
15. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis, 2003. 42(4 Suppl 3): p. S1-201.
16. Tonelli, M., N. Pannu, and B. Manns, Oral phosphate binders in patients with kidney failure. N Engl J Med, 2010. 362(14): p. 1312-24.DOI: 10.1056/NEJMra0912522.
17. Bellinghieri, G., D. Santoro, and V. Savica, Emerging drugs for hyperphosphatemia. Expert Opin Emerg Drugs, 2007. 12(3): p. 355-65.DOI: 10.1517/14728214.12.3.355.
18. Chertow, G.M., S.K. Burke, and P. Raggi, Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int, 2002. 62(1): p. 245-52.DOI: 10.1046/j.1523-1755.2002.00434.x.
19. Block, G.A., et al., Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int, 2005. 68(4): p. 1815-24.DOI: 10.1111/j.1523-1755.2005.00600.x.
20. Block, G.A., et al., Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int, 2007. 71(5): p. 438-41.DOI: 10.1038/sj.ki.5002059.
21. Di Iorio, B., et al., Sevelamer versus calcium carbonate in incident hemodialysis patients: results of an open-label 24-month randomized clinical trial. Am J Kidney Dis, 2013. 62(4): p. 771-8.DOI: 10.1053/j.ajkd.2013.03.023.
22. Chen, N., et al., Sevelamer carbonate lowers serum phosphorus effectively in haemodialysis patients: a randomized, double-blind, placebo-controlled, dose-titration study. Nephrol Dial Transplant, 2014. 29(1): p. 152-60.DOI: 10.1093/ndt/gft232.
23. Vlassara, H., et al., Effects of sevelamer on HbA1c, inflammation, and advanced glycation end products in diabetic kidney disease. Clin J Am Soc Nephrol, 2012. 7(6): p. 934-42.DOI: 10.2215/cjn.12891211.
24. Navarro-Gonzalez, J.F., et al., Effect of phosphate binders on serum inflammatory profile, soluble CD14, and endotoxin levels in hemodialysis patients. Clin J Am Soc Nephrol, 2011. 6(9): p. 2272-9.DOI: 10.2215/cjn.01650211.
25. Caglar, K., et al., Short-term treatment with sevelamer increases serum fetuin-a concentration and improves endothelial dysfunction in chronic kidney disease stage 4 patients. Clin J Am Soc Nephrol, 2008. 3(1): p. 61-8.DOI: 10.2215/cjn.02810707.